In 1997, McPherron et al. discovered a protein that was intimately involved in regulating muscle mass [1]. They published their results in the prestigious journal Nature and to date it has been cited thousands of times in the literature. The protein they discovered belongs to the transforming growth factor beta (TGF-β) superfamily of proteins and they named it GDF-8, which is now widely known as myostatin.
The researchers had created mice that lacked myostatin by disrupting the gene for it. These mice would grow into adulthood with immense muscle mass. Their individual muscles weighed 2-3x more than those of their normal counterparts. Histological analysis revealed that the marked increase in muscle size was due to both hypertrophy (increase in muscle fiber CSA) and hyperplasia (increase in muscle fiber number).
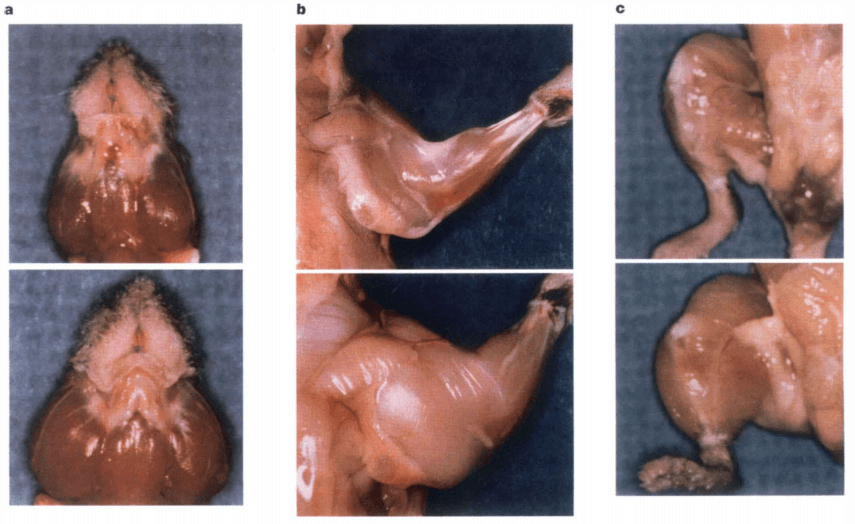
You can imagine how exciting it is when you find a single gene with such a profound effect — without any obvious side effects. This paves the way for drugs that can target the protein itself (or its signaling pathway) in order to treat muscle-wasting diseases or muscle dystrophy. Naturally, these drugs can be of interest for those looking to enhance their physique as well.
Myostatin plays an important role in muscular development across species, including humans
Of course, the question was how universal this finding was. Are mice unique snowflakes, or does myostatin play a crucial role in muscular development across species? If it does, it might hold promise for humans well. Indeed, it didn’t take researchers long to figure out that the Belgian Blue cattle, famous for their muscular and lean appearance, thank their appearance to a naturally occuring mutation in the myostatin gene [2].
The same thing can be seen in other animals, but most importantly, a paper published in 2004 was strongly suggestive that this also happens in humans [3]. In this paper, an extraordinarily muscular boy was described. The cross sectional plane of his quadriceps muscle was roughly twice as thick as that of regular children. Additionally, the subcutaneous fat pad was half as thick as that of regular children. Moreover, the kid was pretty damn strong as well. The authors write: “Now, at 4.5 years of age, he continues to have increased muscle bulk and strength, and he is able to hold two 3-kg dumbbells in horizontal suspension with his arms extended.”. That’s insane for a 4-year-old.
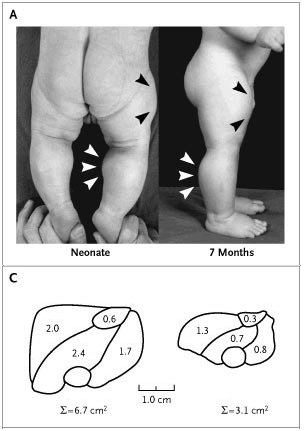
Genetic analysis revealed that he had a mutation in both alleles of the myostatin gene. Interestingly, his mother had a mutation in one of the alleles and coincidentally was reported to be a former professional athlete. Unfortunately, the father remains unknown. Either way, these results pretty much confirm that myostatin is also a strong negative regulator of muscle mass in humans. And if you knock it out, you get big, basically.
Fast-forward over two decades: where are the myostatin inhibitors?
Well over 2 decades after the initial discovery of myostatin, and 16 years after this case study was published, you might wonder: “where the F*** are those myostatin inhibitors to make me big and strong?” Good question! Did the pharmaceutical industry overlook these findings? Of course not! However, myostatin inhibitors aren’t as awesome (yet) as we have hoped them to be. A review paper published this month narrates this out [4].
While animal research has shown effectiveness of myostatin inhibition in a variety of ways, clinical trials so far have been rather disappointing. A total of 6 randomized placebo-controlled trials have been conducted by the pharmaceutical industry in patients with muscular dystrophy . These trials can be found here:
https://clinicaltrials.gov/ct2/show/NCT02310763 (PF-06252616 by Pfizer)
https://clinicaltrials.gov/ct2/show/NCT03039686 (RO7239361 by Roche; two trials)
https://clinicaltrials.gov/ct2/show/NCT01099761 (ACE-031 by Acceleron Pharma)
https://clinicaltrials.gov/ct2/show/NCT02927080 (ACE-083 by Acceleron Pharma)
https://clinicaltrials.gov/ct2/show/NCT00104078 (MYO-029 by Wyeth [now Pfizer])
These trials examined pediatric as well as adult patients with muscle dystrophy, different routes of administration, different trial durations and different outcome measures. With the exception of one trial (the one using ACE-083), there were no, or very small, increases in muscle mass. Importantly, none of the trials demonstrated functional efficacy — which obviously is of great importance when treating muscular dystrophy. In other words: the patients seemingly didn’t get stronger.
The one exception, ACE-083, showed an increase in muscle mass of the biceps of about 15 % and of the tibialis anterior of about 20 % in a dose-escalation study in the group that received the highest dosage. However, no functional efficacy was found. Although this could’ve been attributed to their method of testing, which involved multiple muscle groups while they only injected it into a single muscle (and this drug works locally, so that’s pretty important).
Myostatin inhibitors in clinical trials with healthy subjects
What about trials with healthy subjects? A lack of results in muscular dystrophy doesn’t imply a lack of results in healthy individuals. Unfortunately data about this is quite scarce and not that promising either.
Subjects were given 3 doses of PF-06252616 (10 mg/kg bw) on day 1, 15, and 29 [5]. Lean body mass was assessed with DXA imaging, and regionally muscular hypertrophy was assessed with an MRI scan of the thigh. There was no difference in whole-body lean mass as measured by DXA when compared to placebo. There was, however, a significant increase in thigh muscle volume as assessed using MRI of roughly 5 %. To put that in perspective, thigh muscle volume increased by 6.3 % in young men receiving 125 mg testosterone enanthate weekly for 20 weeks (without concurrent resistance exercise) [6]. Such a dosage is only slightly above what is used for testosterone replacement therapy (75-100 mg weekly) [7]. Needless to say those results don’t really blow me away. However, it would be interesting to see how these results would develop over a longer period of time and with concurrent resistance training. Importantly, a recent paper concludes that the drug “was generally safe and well tolerated in patients with DMD” [8]. This trial lasted almost a year and had a good sample size. But, of course, the sample consisted of boys with duchenne muscular dystrophy. The side effect profile might differ in (healthy) adults. (Note that this trial did conclude that it lacked efficacy in this disease.)
Was the dosage of PF-06252616 too low perhaps? Well, before they performed the experiment with repeated doses of 10 mg/kg, they had performed an experiment with single ascending dose levels between 1 and 40 mg/kg bw: 1, 3, 10, 20, and 40 mg/kg bw. The 10 mg/kg bw dose actually led to the largest increase in lean body mass:
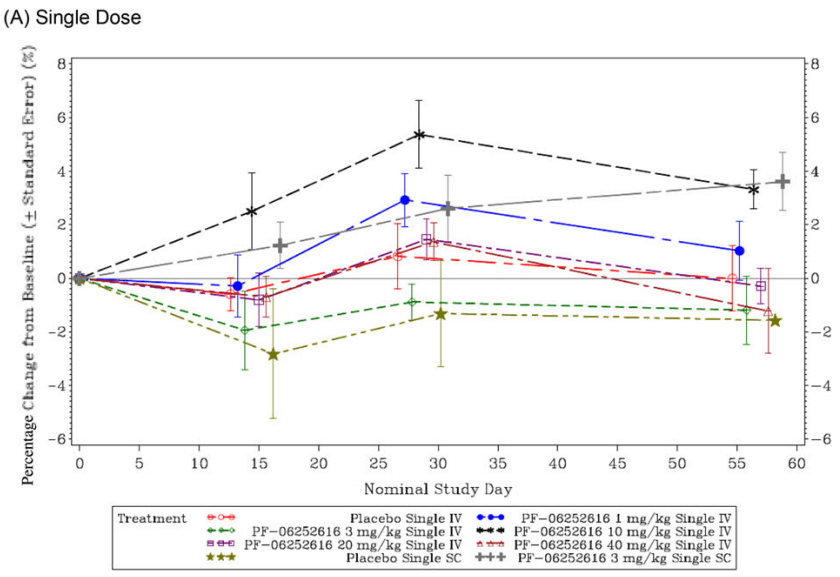
These results make it less likely that the dosage was too low (one could argue, perhaps, that the frequency of injection was too low). The results nevertheless seem disappointing.
Similar results were seen in healthy postmenopausal women receiving ACE-031 [9]. The women were randomized into groups receiving different doses of ACE-031 (0.02, 0.05, 0.01, 0.3, 1, and 3 mg/kg bw). Thigh muscle volume increased by 5.1 % in the group receiving 3 mg/kg bw. Notably, FSH decreased substantially (this hormone is greatly increased in postmenopausal women). ACE-031 is a protein that resembles the extracellular domain of the ActRIIB receptor. As such, it binds ligands for this receptor, including myostatin, but also activin. Activin is a hormone that stimulates FSH production. Conversely, ACE-031 inhibits activin action and thus decreases FSH. Perhaps these nonspecific actions of ACE-031 on proteins other than myostatin can explain some of its side effects that have halted clinical research:
“The adverse events that the trial participants experienced — minor nose and gum bleeding and dilation of blood vessels in the skin — were not, in and of themselves, considered dangerous. However, the companies and regulatory agencies involved say they need to fully understand these events before continuing clinical studies of ACE-031.” [10]
ACE-083 yielded some more exciting results in healthy postmenopausal women [11]. The injections were given intramuscular into the rectus femoris and led to an increase in volume of 14.5 % of this muscle 3 weeks after the last dose of 2x 200 mg. A 8.9 % increase was seen after 3 weeks after injections into the tibialis anterior. Importantly, virtually no change was noted in the contralateral muscle that didn’t receive the injections. As mentioned earlier, this drug acts locally, which makes it less practical. On the other hand, it’s likely to improve its safety profile — after all, there’s limited systemic exposure. Indeed, little difference was noted in adverse events between those treated with placebo and those with ACE-083. Unfortunately, and also the reason why this drug got discontinued later, no changes in strength were observed.
Conclusion
The problems with the clinical trials and these drugs so far are varied. Trials lacking sufficient statistical power to detect changes, side effects occurring because of nonspecific action of the drug in some of them, action limited locally which limits its practical use, and plainly the lack of efficacy in muscle dystrophy in general. This makes it less appealing for the pharmaceutical industry to further pursue. Nevertheless, the drugs can still be effective in healthy individuals. If I look at PF-06252616 (also known as domagrozumab) for example, this drug seems to hold potential in healthy individuals. But say, a trial lasting 20-week in healthy individuals performing resistance exercise, is obviously lacking. It would be really interesting to see what it does under such conditions and how it performs efficacy and safety-wise compared to for example 600 mg of testosterone enanthate weekly.
Either way, after all these years, research hasn’t led to the awesome results that would be expected based on animal models (and the muscular kid with the myostatin gene mutation).
References
- McPherron, Alexandra C., Ann M. Lawler, and Se-Jin Lee. "Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member." Nature 387.6628 (1997): 83-90.
- Kambadur, Ravi, et al. "Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle." Genome research 7.9 (1997): 910-915.
- Schuelke, Markus, et al. "Myostatin mutation associated with gross muscle hypertrophy in a child." New England Journal of Medicine 350.26 (2004): 2682-2688.
- Wagner, Kathryn R. "The elusive promise of myostatin inhibition for muscular dystrophy." Current Opinion in Neurology 33.5 (2020): 621-628.
- Bhattacharya, Indranil, et al. "Safety, tolerability, pharmacokinetics, and pharmacodynamics of domagrozumab (PF‐06252616), an antimyostatin monoclonal antibody, in healthy subjects." Clinical pharmacology in drug development 7.5 (2018): 484-497.
- Bhasin, Shalender, et al. "Testosterone dose-response relationships in healthy young men." American Journal of Physiology-Endocrinology And Metabolism (2001).
- Bhasin, Shalender, et al. "Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline." The Journal of Clinical Endocrinology & Metabolism 103.5 (2018): 1715-1744.
- Wagner, Kathryn R., et al. "Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy." Neuromuscular Disorders (2020).
- Attie, Kenneth M., et al. "A single ascending‐dose study of muscle regulator ACE‐031 in healthy volunteers." Muscle & nerve 47.3 (2013): 416-423.
- https://www.mda.org/quest/article/update-ace-031-clinical-trials-duchenne-md (Accessed on 15-12-2020)
- Glasser, Chad E., et al. "Locally acting ACE‐083 increases muscle volume in healthy volunteers." Muscle & nerve 57.6 (2018): 921-926.
