Gynecomastia is the benign enlargement of breast tissue in men. It is characterized by a lump extending concentrically from under the nipple. You could describe it as a disk under the nipple. This disk feels firm and rubbery, clearly different from the surrounding tissue.
A lot of men develop gynecomastia during puberty. But this generally resolves itself on its own. However, it's somewhat common in otherwise healthy men too, with about a third having it with a diameter of less than 4 cm [1]. So no worries if you find out you have some gynecomastia: it’s quite normal
Additionally, it's a side effect that can occur with the usage of certain drugs, among which androgenic anabolic steroids (AAS). In this article I will discuss some factors which are involved in the development of gynecomastia (or are commonly said to be so).
Androgens and estrogens
There appears to be consensus in the literature that gynecomastia's primary cause is an imbalance between androgenic and estrogenic action on breast tissue [2]. In a nutshell: estrogens make it grow and androgens inhibit this growth. As such, gynecomastia can develop when there’s a relative or absolute excess of estrogen action or a relative or absolute decrease of androgen action at the breast tissue.
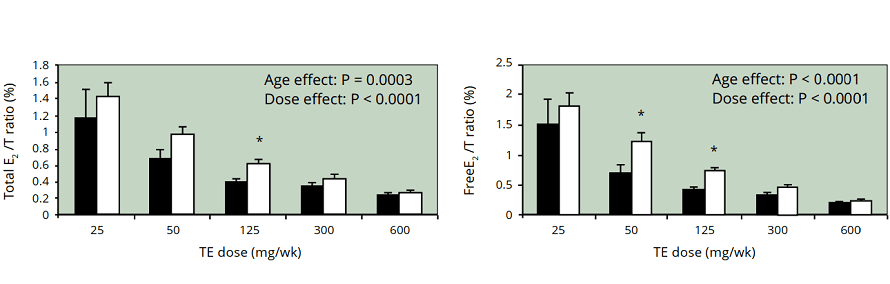
This is why some AAS users might develop gynecomastia. If you inject testosterone, you aren't only increasing the androgen part of the equation. Part of that testosterone will be converted to estrogen by the action of the enzyme aromatase. It is therefor not a rare finding to measure supraphysiological levels of estradiol in the blood of someone who administers testosterone in supraphysiological quantities. In fact, it’s actually quite normal to find increased estradiol levels in such circumstances. However, it should be noted that this does not decrease the androgen to estrogen ratio. Quite in fact, it strongly increases it, as can be shown in the figure below (note that it displays the estradiol to testoserone ratio).
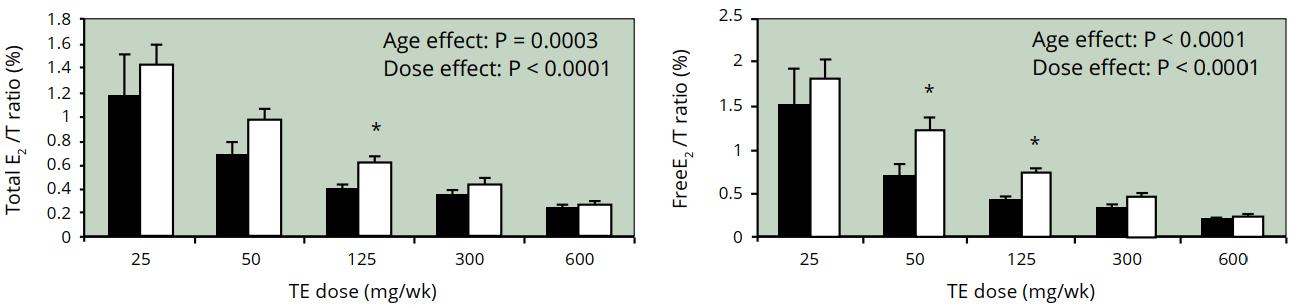
It thus seems unlikely that a decrease in the androgen to estrogen ratio is the cause of gynecomastia during AAS usage. It seems reasonable to assume that in such situations an absolute estrogen excess is the culprit.
After a cycle, however, there will be a transient state of hypogonadism (testosterone deficiency). This means you'll be in an absolute androgen deficiency and also likely a relative estrogen excess. During this period of time you're at an increased risk of developing gynecomastia.
Does prolactin play a role in gynecomastia?
Prolactin is a hormone which seems largely irrelevant in male physiology. However, it can play an important role in pathophysiology. Prolactin is a peptide hormone which is released in large quantities by the pituitary during the final months of pregnancy and postpartum. It is lactogenic, meaning it stimulates milk production of the breasts. Additionally, it has a strong suppressive effect on the secretion of LH and FSH [4]. This is the primary reason why it can be involved in the development of gynecomastia in men. Namely, due to the suppressive effect it has on LH and FSH, it can cause secondary hypogonadism. Resultingly, the androgen to estrogen ratio drops which can lead to gynecomastia.
The direct role of prolactin in gynecomastia, if any, is unclear to say the least. If it does play a role, it seems to be effectively irrelevant in the majority of cases, simply because the prolactin receptor appears to be expressed in breast tissue in only a minority of gynecomastia cases to begin with [5]. It sounds to me like quite a stretch to expect prolactin to do anything in regard to gynecomastia if its receptor isn’t expressed in the breast tissue. Other than that, there appears to be only some speculation based on effects of cross-regulation with growth hormone and progesterone in breast cancer cell lines [6]. Additionally, a study found that incubation of two breast cancer cell lines with prolactin decreased androgen receptor mRNA and binding activity [7]. Nevertheless, if I’ve learned one thing from cancer biology, it’s that they behave awfully different to external signals than regular cells in regard to growth.
On top of that: where would increased prolactin come from during AAS usage? Androgens themselves suppress prolactin secretion. So what remains is conversion to estrogen and that kind of is the thing you’ll be treating anyways when you get gynecomastia. Treatment of gynecomastia is covered in another article.
One other thing I can come up with where it might come from, is the uncontrollable urge of some AAS users to constantly check their nipples for gynecomastia, squeezing and sometimes pinching it. Stop. Doing. That. That has the potential to increase prolactin to some degree too and it can inflame the underlying tissue (which will feel like a disk too). Funnily enough, I stumbled upon some cases in the literature where ’breast selfmanipulation’ apparently had caused galactorrhea (breast discharge) [8].
Does progesterone play a role in gynecomastia?
Progesterone is a steroid hormone and like prolactin it seems to largely play an irrelevant role in male physiology. Again, don’t misunderstand that statement, I’m not saying it does nothing. It does have effects in the male body. Notably in the brain [9].
Anyway, there has been some concern that progesterone might be involved in the development of gynecomastia. First and foremost, AAS won’t really increase your progesterone levels. So there’s that. What remains is that certain AAS have affinity for the progesterone receptor, thereby effectively giving them the potential for progestinic action. Certain AAS have been found to activate the progesterone receptor in a mammalian reporter gene bioassay [10]. Notorious examples are mibolerone (better known under the brand name Cheque Drops) and methyltrienolone, which seem quite potent at it even at low concentrations around 1 nmol/L. Other examples are trenbolone and nandrolone. Other AAS, such as testosterone, stanozolol, oxandrolone, methenolone, and a bunch more, do not activate the progesterone receptor. Well, some do, but only at absurd concentrations you’ll never reach, so practically they don’t.
It’s a bit hard to say if those few AAS I mentioned actually do significantly activate the progesterone receptor in humans too, but at least the bioassay lends some credibility to such claims. But is there then also credibility to the claim that these AAS, via progestenic action, can cause gynecomastia? Progesterone appears to control proliferation and morphogenesis of luminal epithelium of the breast [11]. Or well, at least it seems to do so in women. The luminal epithelium is the cells forming the milk ducts. However, it does not seem to play a role in development of the lobular/glandular tissue (which is responsible for the mass you see/feel with gynecomastia). As such it seems unlikely to play a direct role in the development of gynecomastia. What remains is an indirect role, and it has been proposed that progesterone enhances the effect of estradiol on breast tissue. While I haven’t been able to find human data on this, it at least does not seem to do this in primates [12]. All in all, there does not seem to be any evidence supporting the role of progesterone receptor activation in the development of gynecomastia in AAS users. Indeed, no gynecomastia was noted in a male contraceptive study in which men received 100 mg testosterone enanthate weekly in conjunction with a high dosage of the progestogen levonogestrel (0.5 mg daily) for 6 months [13]. If taking levonogestrel doesn't cause gynecomastia, don't expect some hypothetical fapping to cause it...
References
- F. Q. Nuttall. Gynecomastia as a physical finding in normal men. The Journal of Clinical Endocrinology & Metabolism, 48(2):338–340, 1979.
- H. S. Narula and H. E. Carlson. Gynaecomastia—pathophysiology, diagnosis and treatment. Nature Reviews Endocrinology, 10(11):684, 2014
- K. M. Lakshman, B. Kaplan, T. G. Travison, S. Basaria, P. E. Knapp, A. B. Singh, M. P. LaValley, N. A. Mazer, and S. Bhasin. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. The Journal of Clinical Endocrinology & Metabolism, 95(8):3955–3964, 2010.
- M. De Rosa, S. Zarrilli, A. Di Sarno, N. Milano, M. Gaccione, B. Boggia, G. Lombardi, and A. Colao. Hyperprolactinemia in men. Endocrine, 20(1-2):75–82, 2003.
- M. Ferreira, M. Mesquita, M. Quaresma, and S. Andre. Prolactin receptor expression in gynaecomastia and male breast carcinoma. Histopathology, 53(1):56–61, 2008.
- A. Sansone, F. Romanelli, M. Sansone, A. Lenzi, and L. Di Luigi. Gynecomastia and hormones. Endocrine, 55(1):37–44, 2017.
- C. J. Ormandy, R. E. Hall, D. L. Manning, J. F. Robertson, R. W. Blamey, P. A. Kelly, R. I. Nicholson, and R. L. Sutherland. Coexpression and cross-regulation of the prolactin receptor and sex steroid hormone receptors in breast cancer. The Journal of Clinical Endocrinology & Metabolism, 82(11):3692–3699, 1997.
- R. D. Rohn. Benign galactorrhea/breast discharge in adolescent males probably due to breast selfmanipulation. Journal of Adolescent Health Care, 5(3):210–212, 1984
- M. Schumacher, C. Mattern, A. Ghoumari, J. Oudinet, P. Liere, F. Labombarda, R. Sitruk-Ware, A. F. De Nicola, and R. Guennoun. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Progress in neurobiology, 113:6–39, 2014.
- C. J. Houtman, S. S. Sterk, M. P. Van de Heijning, A. Brouwer, R. W. Stephany, B. Van der Burg, and E. Sonneveld. Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Analytica chimica acta, 637(1-2):247–258, 2009
- Obr, Alison E., and Dean P. Edwards. "The biology of progesterone receptor in the normal mammary gland and in breast cancer." Molecular and cellular endocrinology 357.1-2 (2012): 4-17.
- J. Zhou, S. Ng, O. Adesanya-Famuiya, K. Anderson, and C. A. Bondy. Testosterone inhibits estrogen induced mammary epithelial proliferation and suppresses estrogen receptor expression. The FASEB Journal, 14(12):1725–1730, 2000.
- R. A. Bebb, B. D. Anawalt, R. B. Christensen, C. A. Paulsen, W. J. Bremner, and A. M. Matsumoto. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. The Journal of Clinical Endocrinology & Metabolism, 81(2):757–762, 1996.